culi reaction|Gilman Reagents (Organocuprates): What They're Used For : Manila These reagents were discovered by Henry Gilman and coworkers. [3] Lithium dimethylcopper (CH 3) 2 CuLi can be prepared by adding copper (I) iodide to . 1 dia atrás · Powered by Reelgood. Jenna Ortega fans are still waiting for a second season of Wednesday (the show was renewed for Season 2 in January 2023) to drop on Netflix. .
0 · Organocuprates (Gilman Reagents): How They’re Made
1 · Gilman reagent toward the synthesis of natural products
2 · Gilman reagent
3 · Gilman Reagents (Organocuprates): What They're Used For
4 · Gilman Reagents
5 · Gilman Reagent: Preparation and reactions with easy mechanism
6 · Gilman Reagent & Organocuprates
7 · Ch18: Organocopper reagents
8 · 19.13: Conjugate Nucleophilic Addition to α, β
9 · 19.13 Conjugate Nucleophilic Addition to α,β‑Unsaturated
Foi embasado no Plano de Dados Abertos da UFFS de Janei.
culi reaction*******Gilman reagents have some useful contrasts to Grignard reagents. First of all, they can be used to perform conjugate addition reactions (“1,4-addition”) on alpha,-beta unsaturated ketones. In contrast, Grignard reagents tend to only add to the carbonyl carbon. .
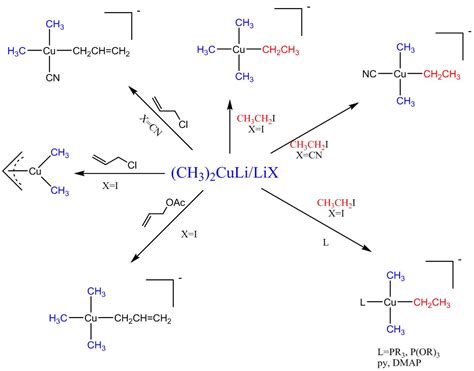
These two modes of reaction are referred to as 1,2-addition and 1,4-addition respectively. A 1,4-addition is also called a conjugate addition. Basic Reaction of 1,4 Conjugate .These reagents were discovered by Henry Gilman and coworkers. [3] Lithium dimethylcopper (CH 3) 2 CuLi can be prepared by adding copper (I) iodide to .Gilman reagents (organocuprates, often written as “R 2 CuLi” are not made the same way as Grignard or organolithium reagents. Instead of being made from alkyl halides, they .Conjugate addition of an organic group is carried out by treating the α,β-unsaturated ketone with a lithium diorganocopper reagent, R 2 CuLi. As we saw in Section 10.7, lithium .

Gilman reagents, also known as organocuprates, are organometallic compounds containing copper, lithium, and two R groups, which may be alkyl or aryl. They have the general .
culi reaction Gilman Reagents (Organocuprates): What They're Used ForThis organic chemistry video tutorial provides a basic introduction into the Gilman reagent also known as an organocuprate. It discusses how to synthesize t.
Stereochemistry of Gilman Reagent. The coupling reaction with allylic substrates usually proceeds via an SN 2-type reaction with high selectivity for the γ-position.. The coupling .Conjugate Addition with Organocopper reagents. Reaction type : Nucleophilic Addition. Summary. Organolithium cuprates, R 2 CuLi are particularly useful for conjugate or 1,4 .
Gilman Reagents (Organocuprates): What They're Used ForGilman reagent is mainly based on RLi and CuX or R 2 CuLi with THF or Et 2 O as solvent. In general, the temperature range for the Gilman reaction is between −78 °C to 0 °C. .
Stereochemistry of Gilman Reagent. The coupling reaction with allylic substrates usually proceeds via an SN 2-type reaction with high selectivity for the γ-position.. The coupling reaction of Gilman reagent (Ph 2 CuLi) with 2-bromobutane proceeds via retention of configuration but the same reaction with 2-iodobutane .Importance of Corey-House Synthesis. This method is a better method than the Wurtz reaction. An alkyl halide and a lithium dialkyl copper are reacted to give a higher hydrocarbon. R’-X + R2-CuLi ⇾ R-R’ + R-Cu + LiX. (R and R’ may be same or different) For example, CH3CH2CH2Br + (CH3)2CuLi ⇾ CH3CH2CH2CH3 + CH3Cu + LiBr.
Conjugate Addition Reactions. In 1941, Kharash discovered that Grignard reagents add to cyclohexenone in presence of Cu(I) resulting in 1,4-addition instead of 1,2-addition. This work foreshadowed extensive studies on the conjugate additions to enones with organocuprates. Note that if a Grignard reagent (such as RMgBr) is used, the reaction .culi reactionAlkane synthesis using R. 2. CuLi. Reaction type: " Nucleophilic Substitution ". Summary. Organolithium cuprates, R2CuLi, react with alkyl halides forming a new C-C, giving alkanes. Primary alkyl iodides make the best substrates otherwise elimination can be a problem. The R group of the cuprate can also be aryl or vinyl.Question: Determine the product of the following reaction sequence. Culi 2. Hz0+ 3. KMnO4/NaOH 0 0 0 0 0 OH The reaction, however, is slightly different as the alkyl groups add to the carbon of the double bond rather than to the one of the carbonyl: This is called 1,4-addition unlike, for example, the Grignard reaction which is a classical 1,2-addition. This has to do with the “hard” and “soft” acids and bases concept, and we will dedicate a .Alkane synthesis using R. 2. CuLi. Reaction type: " Nucleophilic Substitution ". Summary. Organolithium cuprates, R2CuLi, react with alkyl halides forming a new C-C, giving alkanes. Primary alkyl iodides make the best substrates otherwise elimination can be a problem. The R group of the cuprate can also be aryl or vinyl.One particularly valuable reaction of alkyllithiums occurs when making lithium diorganocopper compounds, R 2 CuLi, by reaction with copper(I) iodide in diethyl ether as solvent. Often called Gilman reagents (LiR 2 Cu) , lithium diorganocopper compounds are useful because they undergo a coupling reaction with organochlorides, bromides, and .One particularly valuable reaction of alkyllithiums occurs when making lithium diorganocopper compounds, R 2 CuLi, by reaction with copper(I) iodide in diethyl ether as solvent. Often called Gilman reagents (LiR 2 Cu), lithium diorganocopper compounds are useful because they undergo a coupling reaction with organochlorides, bromides, and . Gilman reagent is mainly based on RLi and CuX or R 2 CuLi with THF or Et 2 O as solvent. In general, the temperature range for the Gilman reaction is between −78 °C to 0 °C. The electronic and steric effects of the R group directly affect the regioselectivity of the products formed as a result of the Gilman reaction.
Conjugate Addition with Organocopper reagents. Reaction type : Nucleophilic Addition. Summary. Organolithium cuprates, R 2 CuLi are particularly useful for conjugate or 1,4-addition to α,β-unsaturated aldehydes and ketones. Lithium dialkylcuprates are formed from organolithium compounds ()Other organometallic reagents such as alkyl lithiums tend to .Gilman reagent is an organocuprate reagent consisting of lithium, copper, and alkyl group with the molecular formula [R-Cu-R] + Li – (lithium dialkylcuprate). It is used to synthesize new compounds consisting of carbon-carbon bonds from alkyl, aryl, and vinyl halides. Typically, the SN2 reaction method is employed to obtain the final product.
30 de abr. de 2022 · CENTRO. A CAMPINEIRA LOTERIAS. Av Doutor Moraes Salles, 1208 – Centro. Telefone: (19) 3233-6034. A PREDILETA LOTERIAS. R General Osório, 907- .
culi reaction|Gilman Reagents (Organocuprates): What They're Used For